First evidence of the effectiveness of a field application of RNAi technology in reducing infestation of the mite Varroa destructor in the western honey bee (Apis mellifera)
First evidence of the effectiveness of a field application of RNAi technology in reducing infestation of the mite Varroa destructor in the western honey bee (Apis mellifera)
Abstract
Background
The mite Varroa destructor is the most serious pest of the western honey bee (Apis mellifera) and a major factor in the global decline of colonies. Traditional control methods, such as chemical pesticides, although quick and temporarily effective, leave residues in hive products, harming bees and operators’ health, while promoting pathogen resistance and spread. As a sustainable alternative, RNA interference (RNAi) technology has shown great potential for honey bee pest control in laboratory assays, but evidence of effectiveness in the field has been lacking.
Methods
We investigated the efficacy and feasibility of a RNAi treatment to improve bee health under natural beekeeping conditions by integrating a honey bee diet with a mixture of dsRNA targeting V. destructor acetyl-CoA carboxylase, Na+/K+ ATPase and endochitinase genes.
Results
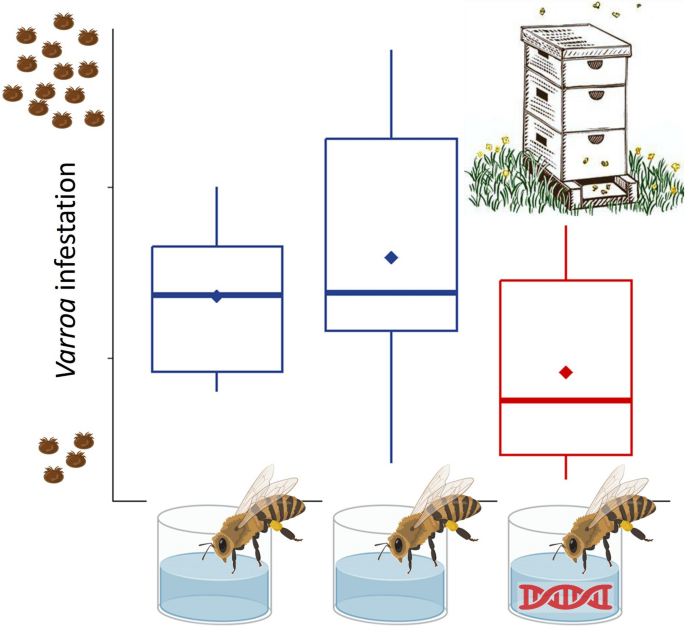
In treated hives, we observed that the average infestation rate of phoretic Varroa mite was reduced by 33% and 42% relative to control bees fed with sucrose and GFP-dsRNA, respectively. The dsRNA treatment did not affect bee survival, and the beekeepers involved in the project found the method manageable in the apiary and non-intrusive to production activities.
Conclusions
Our findings demonstrate the feasibility and effectiveness of RNAi technology in reducing Varroa mite infestations under natural rearing conditions. This study supports the potential of RNAi as a promising alternative to chemical pesticides, offering a targeted, efficient and sustainable solution for managing V. destructor in honey bee populations.
Graphical Abstract
Background
The ectoparasitic mite Varroa destructor is the most serious pest of the western honey bee, Apis mellifera, worldwide. No other pathogen or parasite has had a comparable impact on this species, in part because V. destructor only recently adapted from its original host, the Asian honey bee Apis cerana, to exploit a new host with very limited innate defences. Contrary to what occurs in A. cerana colonies, in A. mellifera the parasite infests both the drone brood and the more persistent worker brood, leading to higher infestation levels [1]. The mite feeds on fat bodies of adult bees during its dispersing phase and primarily on haemolymph of bee pre-imaginal stages during the reproductive phase [2]. This causes several injuries, such as reduction of body weight in hatching bees, a deficit in sperm production in drones, alteration of flying, homing and orientation abilities in foragers, and downregulation of honey bee’s immune response [3]. Varroa destructor is also a viral reservoir and the main transmitter of some honey bee-associated viruses like Deformed wing virus (DWV). Although bee viruses usually persist as unapparent infections, under certain stressful conditions they can dramatically cause serious or lethal disease in individual bees or the collapse of entire colonies (e.g. [4]).
In addition, several studies have shown that V. destructor can interact with other biotic and abiotic stressors, such as environmental factors, other parasites and pesticides, leading to an even more serious impact on honey bee health [5,6,7]. Therefore, V. destructor is considered the major driver of honey bee colony decline around the world, with important economic losses in the beekeeping sector, due to both the lack of production and the increase in the costs necessary for treatments [8]. If mite populations remain undetected and untreated, infested honey bee colonies usually collapse within 1 to 3 years [1, 9].
To treat mite infestation, beekeepers have relied mainly on synthetic acaricides, such as formamidines, organophosphates and pyrethroids, because they are generally easy and fast to use and generally very effective. However, their effectiveness has decreased in recently because of their extensive use, resulting in the evolution of the mite’s resistance in apiaries from several countries [10, 11]. Moreover, these acaricides generate residues that accumulate in beeswax, bee bread and honey, and they can be transferred to brood and adult honey bees, with negative effects on the colony's health [12,13,14].
Because of the adverse impact that synthetic acaricides have on bees and bee products worldwide, beekeepers are increasingly using non-hard chemical control methods, like essential oils and organic acids, which are usually less efficient compared with synthetic acaricide treatments but still effectively able to control mite populations [15]. Organic acids are naturally found in bee products and have a lower risk of triggering resistance in mites [16] but can nonetheless have some negative effects on bees, such as decreasing worker populations, increasing capped brood removal or decreasing drone sperm quality [17].
As set out in the “Farm-to-fork” initiative, a strategy aiming at accelerating the European transition towards a sustainable food system, the European Commission has adopted measures to reduce by 50% the overall use of synthetic pesticides and the resulting risk by 2030, at the same time promoting greater use of alternative methods of protection from parasites and diseases (European Green Deal 2020). Therefore, it is crucial to develop alternative approaches to treating V. destructor that do not generate resistant populations of mites and are safe for bees, bee products, beekeepers and the environment.
Utilisation of RNA interference (RNAi), an intracellular mechanism of sequence-specific gene silencing conserved across eukaryotes, has been proposed as a targeted and sustainable pest-control strategy, in particular in agriculture [18]. RNAi-based technologies for pathogen or pest control exploit this pathway to suppress the expression of specific gene transcripts through the delivery of sequence-specific dsRNA complementary to mRNA transcripts that encode for proteins important for the survival or reproduction of the target organism [19]. dsRNAs are emerging as a potential alternative to synthetic pesticides, because their sequence-dependent mode of action makes them more selective, efficient and flexible compared to other conventional agrochemicals. Besides, dsRNAs generally have limited environmental persistence in soil, sediment and water and do not affect human health [20, 21].
Over the last decade, research has explored the efficacy of RNAi in the control of several common honey bee pathogens and parasites including viruses like DWV [22, 23], Israeli acute paralysis virus (IAPV; [24, 25]) and Sacbrood virus (SBV; [26, 27]), the microsporidian Vairimorpha ceranae (formerly, Nosema ceranae; [28, 29]) and the small hive beetle Aethina tumida [30].
Laboratory studies on V. destructor have shown that injection of dsRNA or soaking of the mites into a dsRNA solution can result in significant gene silencing, although the efficacy depends on the target gene [31,32,33]. As an alternative, feeding bees with a syrup supplemented with dsRNAs that target mite genes resulted in effective uptake by mites, in turn reducing their survival [34] or fertility [35]. Recently, a symbiotic bacterium from honey bee gut was engineered to repeatedly produce dsRNA against genes essential for V. destructor metabolism and was successfully fed to the bees. Mites on bees nourished with the engineered bacteria had a reduced survival rate compared with mites feeding on control bees [23].
These promising results were all obtained under controlled conditions, but no field trials were carried out. In contrast to laboratory experiments, where dsRNA effects are tested in isolation and on small scales, open field environments present physical and biological parameters which are largely unpredictable and highly dynamic.
We investigated the efficiency and feasibility of an RNAi treatment to improve bee health under natural beekeeping management. We integrated western honey bee diet with dsRNAs against V. destructor gene sequences to determine whether this reduces the parasite load in field conditions.
This research is part of the project “BeeOShield”, funded by Rural Development Program for Veneto region 2014–2020 (Measure 16), a European Union instrument that allows member states, and in this case the individual Italian regions, to support increasing innovation in agriculture and forestry-related activities. Specifically, projects funded under Measure16 are expected to be experimental research aimed at an immediate follow-up on agriculture and forestry practice to be developed in cooperation with stakeholders. This is motivated by the need to fill the counterproductive lack of effective interactions between researchers and practitioners in this field, especially in Europe.
In this context, we developed the project together with the beekeepers managing the apiaries involved in the trial, adapting the experimental protocol to their production needs, involving them directly in the administration of the dsRNA and gathering their feedback and suggestions. This approach, despite having imposed some limitations on data collection (like, for instance, allowing direct measurement of phoretic infestation levels only, see below), allowed us to evaluate the feasibility of the transition from laboratory to field of the RNAi-based technology on Varroa mite control. The positive results of the present work, while preliminary, encourage further developing and enhancing these techniques of honey bee management.
Methods
Target gene selection and dsRNA synthesis
Genes to be silenced were chosen among the targets of acaricide compounds that reduce survival of mites by inhibiting gene function.
Acetyl-CoA-carboxylase (ACC) is an enzyme that plays a fundamental role in fatty acid metabolism. The tetronic/tetramic acid family of acaricides inhibit ACC binding to the carboxyltransferase domain, thus interfering with the biosynthesis of lipids in insects and mites [36].
Na+/K+ ATPase is a membrane-bound enzyme responsible for ion transport which has an important role in the regulation of membrane permeability and osmotic balance. This ATPase is the target of some defensive compounds produced by plants, such as pyrethrins and cardiac glycosides, which exhibit strong toxicity against insects and mites [37, 38].
Chitinases (CHITs) are enzymes involved in chitin degradation and reconstruction during the process of arthropod moulting. Some acaricides such as diflubenzuron and scopoletin interfere with the expression of chitinase genes and thus prevent mites from undergoing normal growth and development [39].
A 248-bp dsRNA (VdACC-dsRNA) was designed in the carboxyltransferase domain from the V. destructor acetyl-CoA carboxylase mRNA sequence (XM_022805405); a 249-bp dsRNA (VdATPase-dsRNA) was designed from the V. destructor Na+/K+ ATPase mRNA sequence (XM_022791887) and a 211-bp dsRNA (VdChit-dsRNA) was designed partially in the glycosyl hydrolase 18 conserved domain from the V. destructor endochitinase mRNA sequence (XM_022796590). All sequences were retrieved from the NCBI database. Since using RNAi for mite control requires that it does not negatively affect honey bee health, we compared the sequences of the three candidate dsRNAs with the A. mellifera genome to prevent off-target bee gene silencing. The dsRNA for the green fluorescent protein (GFP-dsRNA, 432 bp), which served as a negative control, was taken from previous studies [22, 24]. dsRNA sequences are available in Additional file 1: Text S1.
The large quantity of dsRNA was synthesized in vitro by AgroRNA (Genolution, Seoul, South Korea), shipped in distilled water at ambient temperature and kept at –20 °C until use.
Effectiveness of dsRNA treatment in the laboratory
Administration of dsRNA by soaking mites
Varroa mites were collected from highly infested hives of one of the apiaries (TV6; Additional file 1: Table S1). Adult mites were dislodged from adult honey bees with powdered sugar and rinsed with water, and 30 mites were randomly assigned to each of four treatment groups: (i) VdACC-dsRNA, (ii) VdATPase-dsRNA, (iii) VdChit-dsRNA and (iv) control group, with five biological replicates for each one. They were placed in 500 μl microfuge tubes containing 2.5 μg/μl dsRNA (specific of each group) in 0.9% NaCl solution or saline solution only for controls. Mites stayed immersed at 14 °C for 14 h before being removed from the solution, dried and placed separately in Petri dishes for each group and replica, where they were fed on same-age bee larvae at 27 °C and 70% relative humidity. To evaluate the level of target gene expression, surviving mites were sampled from each experimental group at 48 h after the end of the treatment and stored at –20 °C.
RNA isolation and cDNA synthesis
To validate RNAi in soaked mites, total RNA was extracted from a pool of 15–20 mites for each treatment group and replicate. Biological replicates were extracted and analysed separately. Mites were homogenized in 350 μl lysis buffer RA1 (Machery Nagel, Germany) with 5-mm stainless steel beads in a bead mill homogenizer (Tissue Lyser II; Qiagen, Germany) for 2 min at 30 Hz. After centrifugation (5 min, 10,000 × g), the supernatant was used for RNA isolation with Nucleo Spin RNA kit (Macherey Nagel). RNA was eluted into 60 μl RNase-free H2O. After centrifugation, the eluate was applied once more onto the column for a second elution. The yield and purity of the extracted RNA (260/280 and 260/230 nm absorbance ratios) were assessed with a Nanodrop N1000 spectrophotometer (NanoDrop Technologies Inc., USA).
First-strand cDNA was synthetized from 1 µg total RNA using the SuperScript III First-Strand Synthesis System for RT-PCR kit (Invitrogen, USA) following the manufacturer’s protocol.
Primer design and qPCR analysis
The expression of target genes in Varroa mite was quantified with qPCR using a 7500 Real Time PCR System (Applied Biosystem, USA) by Microarray Service (Department of Biology, University of Padova). The employed primers are listed in Table 1.
대화 참여하기